Article Text
Abstract
Background There is a striking latitudinal gradient in multiple sclerosis (MS) prevalence, but exceptions in Mediterranean Europe and northern Scandinavia, and some systematic reviews, have suggested that the gradient may be an artefact. The authors sought to evaluate the association between MS prevalence and latitude by meta-regression.
Methods and findings Studies were sourced from online databases, reference mining and author referral. Prevalence estimates were age-standardised to the 2009 European population. Analyses were carried out by means of random-effects meta-regression, weighted with the inverse of within-study variance. The authors included 650 prevalence estimates from 321 peer-reviewed studies; 239 were age-standardised, and 159 provided sex-specific data. The authors found a significant positive association (change in prevalence per degree-latitude) between age-standardised prevalence (1.04, p<0.001) and latitude that diminished at high latitudes. Adjustment for prevalence year strengthened the association with latitude (2.60, p<0.001). An inverse gradient in the Italian region reversed on adjustment for MS-associated HLA-DRB1 allele distributions. Adjustment for HLA-DRB1 allele frequencies did not appreciably alter the gradient in Europe. Adjustment for some potential sources of bias did not affect the observed associations.
Conclusion This, the most comprehensive review of MS prevalence to date, has confirmed a statistically significant positive association between MS prevalence and latitude globally. Exceptions to the gradient in the Italian region and northern Scandinavia are likely a result of genetic and behavioural–cultural variations. The persistence of a positive gradient in Europe after adjustment for HLA-DRB1 allele frequencies strongly supports a role for environmental factors which vary with latitude, the most prominent candidates being ultraviolet radiation (UVR)/vitamin D.
- Epidemiology
- HLA
- meta-analysis
- multiple sclerosis
Statistics from Altmetric.com
Introduction
It has long been recognised that there is a distinct latitudinal variation in multiple sclerosis (MS) frequency, higher latitude correlating with increased prevalence, incidence and mortalities. Understanding the geoepidemiology of MS can be a valuable source of environmental and genetic aetiological clues. MS geoepidemiology has thus become a major research focus, and the latitudinal gradient hypothesis a point of contention.1–6 While gradients have been demonstrated in Australasia,7 8 Japan,9 Europe10 and North America,11 other studies12 13 have found no association between prevalence and latitude. Also, studies in Mediterranean Europe have found higher-than-expected prevalence for their latitudes, while studies in northern Scandinavia14 have found a lower-than-expected prevalence. This has led some3 4 6 to suggest that the gradient is an artefact.
While individual studies have provided evidence, the only way to evaluate the geoepidemiology of MS is to combine findings from a number of studies, and there have been few of these. Early work by Kurtzke1 described bands of high, medium and low frequency, later revised to vary with longitude.2 However, in a 1994 review of MS epidemiology in Europe15 and a 2001 review globally,3 Rosati argued that the linear gradient hypothesis was an oversimplification, pointing particularly to studies undertaken in Mediterranean Europe after 1980 which found a high prevalence in a Kurtzke medium-prevalence zone,12 and instead proposed that much of the variation in frequency was due to different genetic susceptibilities.
The first meta-analysis of MS geoepidemiology was done by Zivadinov and colleagues in 2003,4 combining data from 69 prevalence and 22 incidence estimates between 1980 and 1998. Importantly, in addition to analysing crude values, Zivadinov age-standardised prevalence, reporting a significant gradient in the crude analysis that was attenuated on age standardisation. The authors reported that no association between latitude and incidence was found after age standardisation, however.
In 2008, Alonso and Hérnan undertook a meta-analysis of MS incidence, including 38 age-standardised incidence estimates between 1966 and 2007.5 These authors found that, in contradiction with the findings by Zivadinov,4 there was a significant association between incidence and latitude, though moderated after 1980.
Recently, Koch-Henriksen and Sørensen6 published findings from a meta-analysis of 97 crude MS prevalence and 122 incidence estimates, reporting ‘modest’ associations between prevalence and latitude in Western Europe and North America. The authors found no association between incidence and latitude within Western Europe or North America. Surprisingly, in Australasia, an archetype of the latitudinal gradient,7 8 the authors reported that there was no association between latitude and prevalence, or incidence after adjusting for study prevalence year.
The systematic reviews of MS prevalence geoepidemiology,4 6 particularly that by Koch-Henriksen and Sørensen,6 had some significant methodological shortcomings that may have influenced their results. Further, in light of our own findings regarding the relationship between latitude and UV/vitamin D and MS risk16 and clinical course,17 18 we sought to re-evaluate the geoepidemiology of MS prevalence using a meta-analysis study design.
Methods
Literature search
We searched PubMed (http://www.pubmed.org), EMBASE (http://www.embase.com) and ISI Web of Knowledge (http://www.isiknowledge.com) for articles matching the keywords ‘multiple sclerosis AND prevalence’ or ‘multiple sclerosis AND epidemiology’ for all publications which could be found up to publication year 2010. In addition, article bibliographies were screened, and some authors referred us to other prevalence studies.
Inclusion criteria
To be included, studies needed to have provided crude and/or age-specific prevalence estimates with definition of the study area, source population and study period. Where this information was not reported, this information was sought from the study authors. The majority of scientific articles were published in English, but also included were articles written in Latin and Cyrillic-based alphabets. Articles were translated by the first author or using online translation software (http://translate.google.com).
Data collection
The following information was abstracted from the study reports: study area, the study prevalence year or final year of a period-prevalence study, the diagnostic criteria used, the source and study populations, and the crude and/or age-specific prevalence data.
HLA analysis
HLA-DRB1 allele frequencies for Europe were obtained from the online database http://www.allelefrequencies.net19 or individual publications.
Statistical analysis
Crude prevalence
Crude prevalence was calculated as the number of prevalent cases ascertained in each study divided by the number of persons in the study population. Where the population size was not reported and was not available from local statistical sources, it was approximated from the reported prevalence estimates and the reported number of cases. The variance of each prevalence estimate was calculated using standard methods.20
Age standardisation
Where age-specific data were available, age-standardised prevalence was calculated by the direct method20 using each of three standard populations: 2009 World, 2009 Australia and 2009 Europe.21 We found no meaningful differences using the different standard populations, and only those for the 2009 Europe population are reported. The variance of each age-standardised prevalence estimate was calculated using standard methods.20
Transformation and study weighting
The prevalence estimates were transformed if necessary to reduce heteroskedasticity for regression analyses.22 For example, age-standardised prevalence estimates were analysed on a logarithmic scale. Each prevalence estimate was weighted by the inverse of its variance, with the variance of transformed estimates approximated using the Delta method.
Meta-regression
Heterogeneity
There was considerable between-study variance in the prevalence estimates, as evidenced by the restricted maximum likelihood estimate of between-study variance, τ2, Cochran's Q-statistic and the I2 statistic. The results for global prevalence (τ2=1.237, Q=3.1×108, p<0.0001, I2=100%), global prevalence with age-specific data (τ2=0.783, Q=6.3×107, p<0.0001, I2=100%) and age-standardised global prevalence (τ2=0.764, Q=4.1×105, p<0.0001, I2=99.43%) were each inconsistent with a shared common effect size.
Because it was not reasonable to assume that all the heterogeneity could be explained by model covariates, random-effects meta-regression models were fitted using STATA/SE for Windows (Version 10.1).
Adjustment for covariates
Covariates were specified a priori, in keeping with our hypothesis that prevalence varies with latitude. Other covariates included prevalence year, the diagnostic criteria used and the inclusion of possible cases.
All regression models included adjustment for prevalence year because, on average, the prevalence estimates increased with time. Most models included a binary covariate for the type of diagnostic criteria used (1=Poser criteria and its variants, 2001 McDonald criteria or 2005 McDonald/Polman criteria, 0=all other diagnostic criteria or studies not specifying or not using systematic diagnostic criteria). In addition, some models included a binary covariate for inclusion of cases classified as possible MS (1=possible cases included, 0=possible cases not included). To improve the fit to the data, some models included a product term formed from the covariates for prevalence year and diagnostic criteria, and a second product-term formed from the covariates for diagnostic criteria and possible cases. The estimates reported are those for the year 2009 and are calculated at the mean levels of the other covariates.
Time-corrected analysis
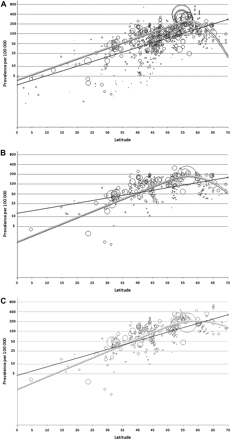
The prevalence estimates depicted in figure 1 for each study (the centres of the circles) are the predicted values from a regression model containing covariates for latitude and actual prevalence year but calculated with prevalence year set at 2009. They are estimates of the values that would have been obtained had each study been conducted in 2009.
Plot of time-corrected prevalence against latitude. (A) All crude prevalence estimates; (B) crude prevalence estimates restricted to those that could be age-standardised; (C) prevalence age-standardised to the 2009 Europe population. The area of each circle is proportional to the inverse of the variance of the prevalence estimates.
Segmented analysis
Examination of the data revealed that the positive association between prevalence and latitude became less pronounced at high latitudes. To accommodate this, segmented models were fitted for supra-regions (global, Western Europe and Europe overall) that non-exclusively included areas located at high latitudes. The segmented models included a covariate for latitude when fitted for latitudes less than or equal to a threshold latitude (L0), and covariates for latitude and its square when fitted for latitudes greater than the threshold. This paper reports the results of a test of the coefficient of the quadratic term. To estimate the threshold value, the segmented model was first estimated by weighted non-linear least-squares minimisation using the PROC NLIN procedure in SAS (Version 9.2). The estimated thresholds for the global model were L0=54.4° (crude prevalence), L0=50.7° (crude prevalence with age-specific data available) and L0=48.8° (age-standardised prevalence).
Adjustment for HLA-DRB1
To assess the contribution of differences in population frequencies of several key MS-associated HLA-DRB1 alleles (HLA-DRB1*15, *11, *01, *03 and *14) to the latitudinal gradient within Europe, linear covariates were added for each allele.
Latitudinal gradient by sex
Sex-specific gradients in age-standardised prevalence with latitude were estimated in a model that included a binary covariate for sex (1=females, 0=males) and a product-term formed from the covariates for latitude and sex. A statistical test of the coefficient of the product-term was used to compare the latitudinal gradients for males and females. Because the age-standardised prevalence estimates had been log-transformed for analysis, this was equivalent to a test of whether the female-to-male ratio of age-standardised prevalence varied by latitude. A test of whether the female-to-male ratio of age-standardised prevalence varied by prevalence year was conducted as a test of the coefficient of a product term formed from the covariates for prevalence year and sex.
Results
Review of literature
Literature searches using the keywords ‘multiple sclerosis AND prevalence’ or ‘multiple sclerosis AND epidemiology’ produced 9379 and 14 808 results respectively. Additional studies were found by searching article references and from author referrals. A total of 365 studies, of which 321 were peer-reviewed, satisfied our inclusion criteria. Only the peer-reviewed studies were used in analyses unless otherwise specified. This provided 650 prevalence estimates, of which 239 could be age-standardised, and 159 of these included sex-specific data. The distribution of all prevalence estimates is depicted in figure 2.
World map showing the distribution of all prevalence estimates included in this meta-analysis.
Information about the studies is summarised in table 1. More detailed information including study area, latitude and prevalence year, diagnostic criteria, and prevalence estimates are shown in supplement 1. Diagnostic criteria used in each study are outlined in supplement 2. Rationales for allocation of study areas to study regions are described in supplement 3. Data on HLA-DRB1 allele frequencies for each study area in Europe for which data could be obtained are shown in supplement 4.
Regional distribution of the 321 studies and their prevalence estimates
Global analyses
Prevalence was significantly (p=0.001) associated with latitude. Restricting the analysis to prevalence estimates that could be age-standardised attenuated the association, but it remained statistically significant (p=0.001) including after age standardisation (p<0.001). On average, the prevalence estimates increased with prevalence year (p<0.001, data not shown). Adjusted for prevalence year, the strength of the association between prevalence and latitude increased in all analyses. Further adjusting for diagnostic criteria and inclusion of possible cases slightly reduced the latitudinal gradient vis-à-vis adjustment for prevalence year alone (table 2).
Estimated change in prevalence/100 000 per degree of latitude showing the effect of adjustment for year of the study, use of systematic diagnostic criteria and inclusion of possible cases
Models allowing a decreasing gradient at high latitudes
A model that allowed additional covariates for latitude and its square to be fitted for high latitudes provided evidence of curvature that was statistically significant (p<0.001) in each prevalence analysis (figure 1).
Table 3 shows the change in prevalence per degree latitude at five latitude degree increments for each of the analysis types. As in table 2, the gradient is most potent when all prevalences are included; the gradient is moderated on restriction to crude prevalence with age-specific data, and enhanced on age standardisation. Also, similar to the trend lines in figure 1, the gradient increases steadily with increasing latitude, reaching a peak around 55°, before changing to a significant inverse gradient above 60°.
Estimated change in prevalence/100 000 per degree of latitude at increments of latitude
Regional analyses
In regional analyses (table 4, figure 3), a statistically significant positive gradient was found within Australasia, the UK region, Atlantic and Central Europe, North America, and Western Europe overall. A statistically significant inverse gradient was found within the Scandinavia and North Atlantic, and Italian regions.
Region-specific associations between latitude and time-adjusted, age-standardised prevalence
Region-specific gradients per degree of latitude for Australasia, Western Europe and North America.
For nations of largely European descent (Europe, Australasia, North America, Latin America excluding the French West Antilles and Israel), the latitudinal gradient in age-standardised prevalence was 3.97 (95% CI 2.27 to 5.66) cases/100 000 per degree of latitude. For all other nations for which we had prevalence data, here defined as non-European descent, the latitudinal gradient was −0.07 (95% CI −1.07 to 0.93) cases/100 000 per degree of latitude, and the difference in trend was statistically significant (p=0.04).
Adjustment for HLA-DRB1
Table 5 shows the effects of adjustment for the frequencies of several HLA-DRB1 alleles on the gradients within Europe. The significant inverse gradient in the Italian region was completely reversed on adjustment, while the positive gradient for Western Europe was almost unchanged and that for Europe enhanced by 33.4%.
Associations between latitude and time-adjusted age-standardised prevalence for the Italian, Western Europe and Europe regions, and with adjustment for HLA-DRB1 frequencies
Latitudinal gradient by sex
The global latitudinal gradients in age-standardised prevalence for males and females were 4.09 (95% CI 2.80 to 5.39) and 7.19 (95% CI 4.84 to 9.53) cases/100 000 per degree of latitude respectively, at the mean global latitude. These estimates were not statistically distinguishable (p=0.358), and hence there was no statistically significant change in the female/male ratio of age-standardised prevalence with latitude. At latitudes up to 59°, the prevalence sex ratio was 2.03 (95% CI 1.71 to 2.42) but without any evidence of significant change with latitude over this range (p=0.768); above latitude 59°, the prevalence sex ratio was 1.59 (95% CI 1.25, 2.02), but again without any evidence of a significant change with latitude over this range (p=0.386).
The prevalence sex ratio did increase over time, increasing from 1.38 in 1949 to 2.34 in 2009, but this did not reach statistical significance (p=0.12) in this sample size. Evaluating the change in prevalence sex ratio within regions revealed no significant change over latitude in any region; however, there was a statistically significant increase in the prevalence sex ratio over time in Australasia (p=0.023) and the UK region (p=0.003).
Exclusion of serial measures
Serial measurements within one location—most commonly in high-prevalence areas of Europe, North America and Australasia—effectively resample the same population if closely spaced in time. To evaluate potential bias, we restricted the analyses to the most recent prevalence estimates for each location and found that this made no material difference to the results (data not shown).
Exclusion of non-systematic diagnostic criteria
Another potential source of bias was the inclusion of studies using non-systematic MS diagnostic criteria. Excluding these studies resulted in no material changes in the estimated associations between prevalence and latitude (data not shown).
Inclusion of non-peer-reviewed studies
Excluded from all analyses thus far were studies (n=47) that were not peer-reviewed. Including them made no material difference (data not shown).
Discussion
This is the most comprehensive meta-analysis of MS prevalence studies yet undertaken, including 650 prevalence estimates from 321 peer-reviewed studies in 59 countries between 1923 and 2009. We found a strong and statistically significant latitudinal gradient for prevalence globally, which persisted on age standardisation and was enhanced on adjustment for prevalence year. The latitudinal gradient was observed only among nations of largely European descent, and while the distribution of HLA-DRB1 alleles did not explain the positive gradient in Europe or Western Europe, adjustment for HLA-DRB1 allele distribution reversed the inverse gradient in the Italian region. Similar gradients were observed for males and females, and the prevalence sex ratio did not change with latitude, or over time.
Exceptions to the gradient
European versus non-European populations
That there was a significant association between latitude and prevalence for European-descent regions, which was absent for regions of largely non-European-descent, suggests the presence of gene–environment interactions. This is not unexpected, given the higher frequencies of high-risk alleles for MS in European populations.23 Interactions between the actual genes (eg, HLA-DRB1*1501) and environmental risk factors (eg, exposure to UV) are likely to exist, and the identification of those interactions is an emerging field of research. Moreover, other aspects such as epigenetic modifications and the timing of exposures further complicate the aetiology of MS.
Italian region
In the Italian region, we observed a significant inverse gradient. On adjustment for all HLA-DRB1 allele frequencies, the inverse gradient was reversed, yielding a positive gradient similar to the rest of Europe. This suggests that the inverse gradient in the region is entirely due to the unique distribution of HLA-DRB1 alleles in this area.
Scandinavia and North Atlantic
Because of a paucity of data on HLA-DRB1 allele frequencies by latitude in the Scandinavia region, we were unable to evaluate their role in this region. Populations in northern Scandinavia are a unique admixture of Swedes, Finns and Sámi.24 While none of the prevalence studies reported large proportions of low-risk groups such as the Sámi in their source populations (11%14 25 to 12%26), it may be that ancestral components from the Sámi contribute to the lower prevalence at these latitudes.
A possible explanation for the inverse gradient in the region was suggested by Kampman and Brustad.27 While latitude correlates with reduced winter UVR and lower vitamin D, in Scandinavia higher latitude does not result in the low levels of serum vitamin D expected owing to high dietary intake. Particularly at the northern latitudes,27–30 dietary consumption of vitamin D in Scandinavia far exceeds that of other European populations, particularly in winter: dietary intake in peninsular Scandinavia ranges from 6.0 to 9.9 μg/day,29–31 while intake in continental Europe is lower, ranging from 2.0 to 3.3 μg/day.32 Thus, despite the absence of vitamin D-generating UV, mean serum vitamin D metabolite (25(OH)D) levels remain close to 50 nmol/l during winter at latitudes up to 71°N.29 31 33 There is now substantial evidence that exposure to UV or vitamin D is associated with MS onset,34 35 and this increased dietary intake of vitamin D could contribute to the region's inverse gradient.
HLA-DRB1 and the gradient in Europe
Importantly, our analysis showed little effect on the latitudinal gradient in Europe after adjustment for the distribution of MS-associated HLA-DRB1 allele frequencies. This is in contradiction with others36 who found that the distribution of HLA-DRB1 accounted for 52% of the variation in prevalence by latitude in Europe, while the UV index accounted for only 31% in univariable analysis. In our analyses, we were able to assign HLA-DRB1 allele frequencies in Europe to a much finer degree than attempted previously,36 finding that adjustment for HLA-DRB1 frequencies increased the latitudinal gradient in Western Europe by 3.5% and the gradient in Europe overall by 33.4%. These findings suggest a strong independent role for non-HLA-DRB1 factors in the gradient in Europe.
Sex and prevalence sex ratio
All trends observed in the total were mirrored in each sex, and no significant difference by sex was observed in any of our analyses. Globally, we found no significant change in the prevalence sex ratio (female/male) across the latitudinal range, nor within the intervals up to and above 59°. We found a 70% increase in the prevalence sex ratio over the 60-year interval for which we have prevalence data, but this did not reach statistical significance in this sample size. These results are different from those reported elsewhere,6 and may reflect the different methods and data included. On examining changes within regions, in no instance did we find a significant change in the prevalence sex ratio over latitude. In some regions, we found an increase in the prevalence sex ratio over time, including significant increases in Australasia and the UK region, while in other regions such as North America, an increase was found but did not reach statistical significance. These regional findings are somewhat in conflict with the significantly increasing prevalence sex ratio over latitude in New Zealand8 and the significantly increasing prevalence sex ratio over time in Canada.37 This disparity may reflect less comprehensive coverage of these regions in our analysis, since a minority of studies provided age and sex-specific prevalence data.
Strengths and improvements from previous studies
This study makes significant improvements upon previous meta-analyses by Koch-Henriksen and Sørensen,6 and Zivadinov,4 and preceding descriptive reviews,1–3 in a number of key elements. These methodological improvements, in both data collection and statistical analysis, provide strong support in favour of our conclusions, and no doubt explain the differential findings from previous studies.
At the most basic, our study is more comprehensive, encompassing a broader range of studies, both geographical and temporal, that satisfy the inclusion criteria. This is due to our use of multiple data sources, as well as our inclusion of studies published in languages other than English, allowing a more powerful evaluation of geographic and temporal changes in prevalence.
Our study improves upon the work of Zivadinov4 in our use of study weighting by the inverse of study variance. As in figure 1 of our paper, there are a number of small outliers, particularly in the crude analysis. If these studies are not weighted in proportion to their small size and high variance, they can potentially affect the interpretation of the associations measured. The study by Koch-Henriksen and Sørensen6 restricted their inclusion of studies, requiring a minimum of 20 cases. While this is one method of addressing variable study quality, this has the effect of moderating any potential gradient, since the number of cases, presuming a constant population, would decrease with decreasing latitude; removing studies with smaller prevalent cohorts would remove a greater proportion of studies at the lower extreme of latitude, biasing the results. Rather as we have done, the use of study weighting by the inverse of study variance would address any potential differences in study quality which might covary with case number, while preserving the maximal study inclusiveness.
A key feature of our study relative to most others1–3 6 is the use of age standardisation. As noted by Zivadinov,4 age standardisation is requisite for studies comparing aggregate-level data from different study regions, which can have significantly different age structures. While some have suggested that age standardisation was unnecessary,6 it made an important difference to the findings of Zivadinov,4 Alonso and Hernán,5 and our own.
Our analyses were strengthened by adjustment for prevalence year, which was significantly associated with prevalence independently of other covariates. Importantly, we observed an association between log-transformed prevalence and latitude prior to time adjustment, which was enhanced on simple adjustment for prevalence year, indicating that our findings are not a statistical artefact of the time-adjustment process. Interestingly, Koch-Henriksen and Sørensen6 also find a significant association between prevalence and prevalence year; however, they do not adjust their analyses of prevalence and latitude for it—doing so in our analysis significantly enhanced the magnitude and significance of the association between latitude and prevalence. Our results show that failure to adjust for prevalence year would underestimate the magnitude and significance of the latitudinal gradient (table 2), particularly when the meta-analysis includes studies over a wide range of time, as was the case here.
A novel feature of our analysis is the segmented, rather than simple, linear models used to evaluate the global gradient: the prevalence gradient increased with latitude, reaching a peak around 55°, before becoming a significant inverse gradient above 60° (figure 1, table 3). This reduction in the gradient at higher latitudes was also observed by Zivadinov et al4; their use of a linear trend to evaluate the significance of the global gradient may contribute to their conclusion of an attenuation after age standardisation, rather than an enhancement as observed here.
For our HLA-DRB1 analyses, we were able to ascribe HLA-DRB1 allele frequencies with a much greater precision than attempted previously. Whereas previous studies36 have evaluated the relationship between prevalence, latitude and HLA-DRB1 at the national or supra-local level, we were able to assign HLA-DRB1 allele frequencies to the majority of the individual prevalence estimates in Europe using HLA-DRB1 surveys within geographically relevant areas. In an area of such genetic complexity as Europe, this is critical in evaluating the role of HLA-DRB1 in the latitudinal gradient.
Study weakness
Several weaknesses of this study need to be borne in mind. First, the analyses are based on prevalence estimates made in different study centres with varying degrees of case ascertainment and different study procedures. Our inclusion criteria excluded case series and other non-population-based estimates of prevalence. However, we did not attempt to grade and select studies for inclusion based on perceived study quality, because to do so requires objective information that is rarely fully reported in MS prevalence studies. Instead, we attempted to take some of the known factors into account in analyses, but we accept that it is not possible to do so completely.
We were not able to remove all residual between-study variance using information available to use on prespecified covariates. This residual variance was most pronounced at the global level; however, in regional analyses (data not shown) the covariates were successful in explaining a much larger part of the between-study variance. This added to confidence that the association of MS prevalence with model covariates—including latitude—is truly reflective of causal factors that correlate with latitude, most particularly environmental factors such as UV/vitamin D.
There are potential sources of bias in our own study procedures, including selection bias from inclusion of serially measured prevalence estimates at the same location or exclusion of non-peer-reviewed studies, and measurement bias from including studies making use of non-systematic diagnostic criteria. However, none of these factors had a material impact on our findings.
A further issue is that of publication bias. We have attempted to address publication bias by drawing our studies from a broad a range of sources, and including studies published in languages other than English, as well as including non-peer-reviewed studies in a subanalysis. Publication bias on the part of the individual study authors is less of a concern than in some other study types, because prevalence studies are necessarily less prone to publication bias by virtue of the absence of a ‘null finding.’ Furthermore, if authors do not pursue publication of findings that are not in marked contrast with previously reported estimates for their area, the published estimates nevertheless capture the regional variance, and the non-published findings would not materially change our conclusions.
Conclusion
We present here the largest and most comprehensive study of MS prevalence yet carried out, finding a significant positive association between latitude and prevalence at the global level, as well as in most regions of European descent. Our findings are inconsistent with preceding reviews of MS prevalence6 34 but in harmony with a methodologically similar meta-analysis of MS incidence.5 Our findings do not concur with all of the conclusions of previous meta analyses4 6; however, we feel that the differences are accounted for by the improved methodologies as described. We feel that the inclusiveness and methodological improvements, particularly age standardisation and time adjustment, indicate that these findings are more representative of the current geoepidemiology of MS than previous studies. European exceptions to the gradient in the Scandinavia and North Atlantic, and Italian regions are explicable by behavioural–cultural and genetic factors that vary geographically within these regions.
In contradiction with work elsewhere,36 HLA-DRB1 variation did not account for the majority of the gradient in Europe, suggesting a greater role for environmental factors that vary by latitude, with the most prominent candidates being UV and vitamin D. No doubt, genetic and environmental factors interact to manifest in the variation in MS prevalence observed, but there are insufficient data available on the distribution of HLA-DRB1 alleles to quantify the proportions precisely. The information gleaned from this demonstration of the existence of a latitudinal gradient for MS prevalence will further the understanding of factors leading to MS and, potentially, help lead to its resolution.
Acknowledgments
We thank the various authors whose published works were requisite for this study, and we would especially like to thank the following authors for their personal correspondences: F Al-Himyari at the Babylon University in Babylon City, Iraq; S Beer at the Klinik Valens Rehabilitation Centre in Valens, Switzerland; J Bentzen at the University of Southern Denmark in Copenhagen, Denmark; A Bhigjee at Inkosi Albert Luthuli Central Hospital in Durban, South Africa; E Celius at Oslo University Hospital in Ullevål, Norway; DAS Compston at the University of Cambridge in Cambridge, UK; N Gryten-Torkildsen at Haukeland University Hospital in Bergen, Norway; H Houzen at Obihiro Kosei General Hospital in Hokkaido, Japan; W Ingram at Peninsula Medical School in Plymouth, UK; J Kurtzke at Georgetown University in Washington, DC, USA; C-H Lai at Changhua Christian Hospital in Changhua, Taiwan; J Larsen at the University of Bergen in Bergen, Norway; R Lonergan at St Vincent's Private Hospital in Dublin, Ireland; E Materljan at the University of Rijeka in Rijeka, Croatia; K-M Myhr at the University of Bergen in Bergen, Norway; A Nicoletti at the University of Catania in Catania, Italy; M Niino at Hokkaido University in Sapporo, Japan; N Robertson at Cardiff University in Cardiff, UK; C Sagnes-Raffy at the Centre Hospitalier, University of Toulouse in Toulouse, France; J Sepčić at the University of Rijeka in Rijeka, Croatia; C Smestad at Oslo University Hospital, Ullevål, Norway; HF Tseng at Kaiser Permanente, Southern California in Pasadena, California, USA; N Tubridy at St Vincent's Private Hospital in Dublin, Ireland; D Williamson at the Division of Reproductive Health of the Centers for Disease Control and Prevention in Atlanta, Georgia, USA; and J Zajicek at the Peninsula Medical School in Plymouth, UK.
References
Supplementary materials
HTML Page - index.htslp
Files in this Data Supplement:
Web Only Data
Files in this Data Supplement:
Footnotes
Funding SSJ is supported by a Menzies Postgraduate Research Scholarship. IVdM is supported by an NHMRC Training Fellowship.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.