Article Text
Abstract
The onset of amyotrophic lateral sclerosis (ALS) is conventionally considered as commencing with the recognition of clinical symptoms. We propose that, in common with other neurodegenerations, the pathogenic mechanisms culminating in ALS phenotypes begin much earlier in life. Animal models of genetically determined ALS exhibit pathological abnormalities long predating clinical deficits. The overt clinical ALS phenotype may develop when safety margins are exceeded subsequent to years of mitochondrial dysfunction, neuroinflammation or an imbalanced environment of excitation and inhibition in the neuropil. Somatic mutations, the epigenome and external environmental influences may interact to trigger a metabolic cascade that in the adult eventually exceeds functional threshold. A long preclinical and subsequent presymptomatic period pose a challenge for recognition, since it offers an opportunity for protective and perhaps even preventive therapeutic intervention to rescue dysfunctional neurons. We suggest, by analogy with other neurodegenerations and from SOD1 ALS mouse studies, that vulnerability might be induced in the perinatal period.
- ALS
- Neurotoxicology
- Neuroepidemiology
- Motor Neuron Disease
- Molecular Biology
Statistics from Altmetric.com
Introduction
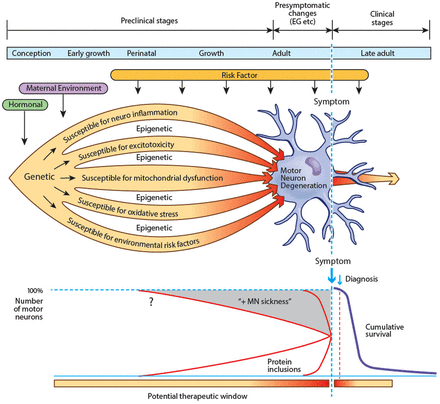
Amyotrophic lateral sclerosis (ALS) appears intractable to therapeutic efforts. This disappointing therapeutic response may simply reflect phenotypic expression triggered much earlier, perhaps decades before onset of clinical symptoms.1 The terms preclinical and presymptomatic are frequently used interchangeably, but here ‘presymptomatic’ refers to the period when there are no clinical correlates, while investigations such as neuro-imaging, electrophysiology or cognitive assessment may be abnormal. ‘Preclinical’ refers to the much longer period when presently there are no identified markers of disease in sporadic ALS (see figure 1).
Biologically, the seeds for the development of amyotrophic lateral sclerosis (ALS) may be sown shortly after conception. Motor neurons and supporting glia are susceptible to many potential insults, such as neuroinflammation, excitotoxicity, mitochondrial dysfunction, excessive oxidative stress and environmental risk factors. Epigenetic influences may further determine individual sensitivity and susceptibility. Environmental risk factors continue to exert their influence throughout life. In combination, these factors cause protein dysfunction and aggregation. Motor neurons and surrounding astrocytes are metabolically stressed, progressively losing function (MN ‘sickness’). After years or decades, cytosolic compensatory mechanisms begin to fail and a clinically identifiable presymptomatic stage starts in which electrophysiological and imaging abnormalities become detectable at a macroscopic level. Finally, the motor system fails and ALS becomes symptomatic and relentlessly progressive. If so, biomarkers may yet become evident throughout the preclinical and presymptomatic stages, thereby enabling the future development of protective or preventive therapeutics (EG, epigenetic effects). The term ‘presymptomatic’ refers to the period when there are no clinical correlates, while investigations such as neuro-imaging, electrophysiology or cognitive assessment may be abnormal. ‘Preclinical’ refers to the much longer period when presently there are no identified markers of disease in sporadic ALS.
Symptom onset in adult neurodegenerations, including ALS, typically occurs in mid-life to late life. In Alzheimer's disease (AD) and Parkinson's disease (PD), pathological changes precede clinical disease by years, if not decades. In both AD2–9 and PD,10–15 there is a lengthy premonitory period before overt features develop.2–9 In PD, there is a period of non-motor precursor symptoms without typical Parkinsonian features during which Lewy bodies may be found during many years before the onset of classic clinical PD.10–15 It has even been suggested that PD may commence in the perinatal period when environmental and genetic influences may lower the threshold of dopaminergic neurons, enabling normal function to continue for decades before the pathophysiological threshold for clinical expression of the disease is exceeded.16
It has previously been suggested that ALS may have a prolonged preclinical period,17–19 but, generally, it is assumed that the clinical onset of ALS is coincident with, or starts shortly after, the onset of the pathological process underlying the disease. Evidence for this relates to a short, presymptomatic period in which there is a reduction in motor unit number estimates and electromyography (EMG) abnormalities.20 ,21 Also, EMG abnormalities are common in clinically strong muscles in overt disease.22
However, absence of detectable change found by these tests of lower motor neuron function does not necessarily equate with normal functioning of anterior horn cells; abnormality of upper motor neuron functioning has clearly been demonstrated to precede clinical deficit in ALS.23 More likely, there is bio-molecular dysfunction at a cellular level that cannot presently be detected, which is insufficient to cause clinical features, but potentially present and building for years or decades prior to onset of clinical disease. In SOD1 ALS mouse models, pathological changes are evident shortly after birth, predating the first clinical abnormalities by 2–3 months. In human genetically-linked ALS (FALS), expression of the disease-causative proteins, or other metabolic defect, must be evident even during embryonic life. Similarly, in sporadic ALS, biological abnormalities reflect a long-lasting morbid process progressing over years, or potentially even decades, before the first symptoms become apparent (see figure 1). Here, we will explore such a possibility in relation to potential early biological changes that might predict the likelihood that ALS will develop in mid-life to late life in susceptible individuals. In this respect, hereditary ALS is a prototype.
Earliest abnormalities in ALS transgenic mice
Rodent models of ALS do not translate faithfully into the human disease, although they do reveal preclinical pathobiological abnormalities.24 ,25 These are relevant in understanding similar early abnormalities in human ALS, especially compensatory mechanisms that delay clinical presentations. Embryonic mutant motor neurons already show morphometric and physiological abnormalities including hyperexcitability.26 Vinsant and colleagues27 ,28 analysed the early changes in the SOD1G93A mouse that included ultrastructural examination of central and peripheral components of the neuromuscular system and correlated these alterations with early muscle denervation, motor dysfunction and motoneuron death. Swollen and vacuolated mitochondria and mega-mitochondria were first observed in the spinal cord 7 days postnatally. These changes were most abundant in motor neuron dendrites but were also found in motor neuron soma, in the presynaptic terminals of neuromuscular junctions and in presynaptic terminals of axo-somatic synapses. Accumulations of small, empty vacuoles in spinal motor neuronal cytoplasm were observed 14 days after birth. These became more numerous by day 30 and later the cytoplasm became full of these vacuoles. By postnatal day 30, there was a significant decrease in axo-somatic type I ‘excitatory’ synapses on the motor neurons and an increase in C-terminals. There was no change in the number of type II ‘inhibitory’ synapses or in the number of total synapses. Clinically, gait alterations and muscle weakness started 30 days after birth and, by 60 days, 20% of motor neurons had undergone degeneration.
Presymptomatic neuronal type-specific degeneration in hSOD1G93A mice involves both spinal motoneurons and corticomotoneurons.29 Interneurons and non-neuronal elements, including glial cells, are also affected in the presymptomatic stage in mutant rodents.30–32 The electrical properties of transgenic rodent ALS motor neurons are abnormal shortly after birth,33–35 with associated synaptic changes, and with alterations in neural circuits and network activity, followed by clinically evident neurological impairment.
Embryonic and perinatal effects
It is likely that there are a number of unidentified risk-genes for sporadic ALS. Development from a single-cell zygote to a mature organism incurs a large number of cell divisions. As a result, mutant genes are frequent during normal development, although most do not have a deleterious effect.36 Inherited mutations, applicable to the 29 nuclear genes that have presently been identified to be associated with hereditary ALS, have the highest risk of disease, since all cells carry the mutant gene.37–42 However, spontaneous gene mutations associated with cell division during embryogenesis and early development are also potentially disease-inducing. Spontaneous mutations blur the difference between hereditary and sporadic ALS.43 ,44 In sporadic ALS, spontaneously arising ‘at risk mutations’, occurring early in development, would carry only a slightly lower risk relative to hereditary ALS.36 Spontaneous mutations arising later in neuronal development have less, or little risk, depending on how early or late in development they occur. How mutant genes translate into ALS clinical phenotypes remains to be elucidated, but it is likely that mutations of only a few of the numerous genes guiding developmental programming and network formation and function will add to the overall burden of risk for developing ALS later in life.45
Neural network development begins at conception, and continues into adolescence and young adulthood.46 However, it is the prenatal and perinatal periods that are associated with the greatest metabolic activity. This is required for neurogenesis, neuronal proliferation and neural differentiation and migration. In humans, most programmed neuron loss (apoptosis) occurs prenatally. The added metabolic demand increases oxidative stress and must be countered by antioxidant production and redox-sensing systems sufficient to control reactive oxygen species (ROS) production, and remove damaged mitochondria.47 During these periods, spontaneous mutations may cause subtle abnormalities in central nervous system (CNS) wiring, connectivity and network formation inducing vulnerability for late-in-life neurodegeneration, including ALS.4 Postnatally, the process of synaptic proliferation continues through middle childhood and is followed by programmed elimination of synapses. Substantial refinement of brain structure and function occurs during adolescence, again a period of potential susceptibility for disease in later life.48
Evolution may not select against early life deleterious mutations that take effect only beyond the reproductive period.49 Although, long life past the reproductive period is important in our species, the ‘grandmother effect’, some genetic traits may exhibit ‘antagonistic pleiotropy’ or phenotypes that improve survival earlier in life, but become deleterious with increasing age.50 Whether this is applicable to ALS has not been determined.
Preclinical genetic, epigenetic and environmental risks
ALS, like other neurodegenerations, is a complex, multifactorial disease with variations in individual susceptibility, age of onset and rate of progression. Genetic and environmental factors that influence susceptibility depend on multiple gene-by-gene and gene-by-environment interactions and epigenetic effects which also drive phenotypic individuality.51 These factors are probably the key to unravelling presymptomatic disease.
Neurodegenerative diseases with Mendelian inheritance and diseases including familial ALS are associated with genetic variants present from the time of conception, even though they do not present clinically until mid to late adulthood. This implies either that these genes are not ‘switched on’ until later life, or that there are decades of progressive cellular compromise eventually culminating in the catastrophic decline manifesting as presentation of clinically overt ALS. Heritability studies suggest that about 60% of the risk of ALS is genetically determined, and the remaining 40% environmentally determined.52 It is unlikely that any environmental contribution to ALS will act in isolation; genetic and epigenetic components must all interact.
Environmental exposure as a risk factor for ALS, although apparently a weak factor in causation of the disease, is likely to be cumulative over time, exceeding a genetic–environmental threshold in those who, some later time, develop ALS. The neurodegenerative process, thereafter, seems to be irreversible and self-perpetuating. A highly penetrant monogenic cause has little requirement for environmental exposure, but in a complex oligogeneic or polygenic disease, such as sporadic ALS, the environmental component is larger.42
The peak incidence of ALS is between the age of 70 and 74 years; thereafter, incidence declines rapidly. In this respect, ALS is different from both AD and PD in which the incidence/prevalence increases with age. The reduced risk in older age is not due to ascertainment bias, and may partly reflect a Gompertzian cohort of people whose susceptibility to ALS is determined by interaction between environmental and genetic risk factors.53–55
Environmental exposure to toxins, smoking, excessive physical activity, occupation, dietary factors and changes in immunity all increase the risk of developing sporadic ALS.53 These factors may drive epigenetic changes over many years, which then induce disease onset and progression. There is a significant association with smoking; prolonged exposure and current smoking increase ALS risk by twofold to threefold.56 ,57 Exposure to pollutants is one mechanism that may trigger and can chronically perpetuate neuroinflammation, but whether repeated low exposure can interact with genetic and epigenetic components in the initiation of ALS is yet to be established.42 Less attention has been directed to more general environmental factors that may trigger the cascade of motor neuron degeneration leading to ALS.58 Nevertheless, neuronal damage from oxidative stress may continue throughout life by accumulation of environmental, occupational, dietary and lifestyle exposures.59 Neuroepidemiological studies of risk factors for ALS suggest that exposure must occur several years before disease onset,43 implying that an environmental trigger may be active for years before clinical disease develops.
Epigenetic changes underlie developmental and age-related biology. Promising epidemiological research implicates epigenetics in disease risk and progression, and suggests epigenetic status depends on environmental risks as well as genetic predisposition. Epigenetics may represent a link between environmental exposures and mechanisms that modify the expression levels of selected genes, without alteration in their DNA sequence. These mechanisms include DNA methylation, histone tail modifications and chromatin remodelling, as well as mechanisms mediated by small RNA molecules. Epigenetic modifications are important because they have similar effects to those of pathogenic mutations, since they are able to silence, increase or reduce the expression of a selected gene in a given tissue.60–62 There is a critical window during development during which such factors can have lasting effects on neuronal gene expression.63
Epigenetic processes have been identified in both AD and PD.64–66 In sporadic ALS, it has been suggested that epigenetic modifications may alter the expression of pathogenesis-related genes leading to the onset and progression of sporadic ALS and ALS-dependent methylation of several genes previously implicated in neuronal development, differentiation and proliferation.67 DNA methyltransferase may be upregulated in motor cortex and spinal cord motor neurons in sporadic ALS.68 Thus, defective epigenetic homeostasis in the CNS, leading to aberrant gene expression, may contribute to CNS dysfunction initiating ALS.61
Excitatory and inhibitory neurotransmission in ALS
The neuron may be rendered hyper-excitable when there are increased glutamate levels, or decreased inhibition, as happens when γ-aminobutyric acid (GABA) activity, the major inhibitory neurotransmitter, is reduced, or a combination of both these. In mutant ALS rodents, excitotoxicity has been documented prenatally and it is recognised preclinically in human sporadic ALS,26 ,69 although it has not been determined how long before clinical symptom onset the excitotoxic state is present.70–72
A hyperexcitable motoneuron would fire more spikes in response to a given synaptic input and consequently more calcium ions would flow into the cytoplasm, eventually leading to neuronal cell death. However, unlike embryonic immature motoneurons, intrinsic hyperexcitability has never been demonstrated in adult motoneurons.73 So the excitotoxicity leading to degeneration in ALS is not caused by changes in the intrinsic electrical properties of the motoneurons themselves. However, excitotoxicity could also be induced by an alteration in the synaptic inputs received by a motoneuron. A reduction of the inhibitory inputs or an increase of the excitatory inputs would lead to higher firing rates, thereby increasing calcium turnover in the cytoplasm.73
Glutamate is critical in the early development of neurite outgrowth and neuronal migration, and the developing brain undergoes a period of increased sensitivity to overstimulation of NMDA receptor channel complexes. GABA appears fundamental to the pathogenesis of ALS.74 In ALS, there is widespread loss of parvalbumin and calbindin-D calcium-binding proteins associated with GABA-ergic interneurons.75 ,76 Decreased inhibition has been shown to occur in ALS motor cortex using magnetic resonance spectroscopy77 and transcranial magnetic stimulation has identified a reduction in short interval intracortical inhibition in asymptomatic carriers of mutations linked to ALS.77 ,78 These studies imply that hyperexcitability precedes the onset of symptomatic ALS.79 In support, positron emission tomography using the ligand flumazenil has identified widespread reduction in cerebral GABA-A receptor binding in ALS.80
A major role of GABA-mediated postnatal transmission is to produce synchronised neural network oscillations.81 ,82 It is presently unresolved if loss of spinal inhibition is a cause or a consequence of segmental neuronal destruction in ALS. Renshaw cell alterations may lead to a hyperexcitable state and eventually motor neuron degeneration. It has been postulated that this hyperexcitability is caused by the loss of the recurrent Renshaw cell-mediated inhibition.83 Alternatively, it is possible that Renshaw cell loss is not an initial causation of motor neuron hyperexcitability and neurodegeneration, but is secondary to motor neuron degeneration.83 ,84
Neuroinflammation
A growing body of data supports the hypothesis that damage induced by different infectious agents may be factors leading to neurodegeneration. This probably acts in synergy with other risk factors, such as ageing, concomitant metabolic diseases and the host's specific genetic signature.85 The immature and preterm brain can be exposed to viral and bacterial infections as well as sterile insults occurring during pregnancy. Such inflammatory episodes presumably usually resolve without harm to the CNS, but nevertheless, may increase vulnerability to neurodegenerative disorders.86 ,87
During development, microglia contribute to the formation of the neural network by stimulating vascularisation and assisting in pruning excess neurons and synapses, as well as facilitating cell differentiation. Throughout life, there is a balance between microglia-derived protective anti-inflammatory cytokines, which are maximum in early development and childhood, versus pro-inflammatory cytokines, which accumulate with ageing and are associated with a chronic inflammatory state.88 A shift toward pro-inflammatory cytokines contributes to increased susceptibility and neurodegenerations.89 Physical aggression in boys during childhood is a predictor of reduced anti-inflammatory cytokines in early adulthood,90 raising the intriguing speculation that the male predominance of ALS might partly be related to reduction of anti-inflammatory cytokines early in life. This may tie in with the findings that patients with ALS have a lower second-to-fourth digit ratio, consistent with higher prenatal circulating levels of testosterone, and possibly a prenatal influence of testosterone on motor neuron vulnerability in later life.91
Mitochondria and mtDNA
Mitochondria are responsible for generating cellular energy, regulating intracellular calcium levels, altering the reduction–oxidation potential of cells and regulating cell death.92 ,93 Mitochondrial dysfunction, an early event in neurodegenerative diseases,93 may serve as a trigger or propagator for neurodegeneration.94 In particular, toxicity from ROS can initiate damage to mitochondrial DNA (mtDNA) leading to respiratory chain dysfunction, which in turn increases the generation of ROS, further facilitating cellular damage, and creating a self-amplifying process.95
The number of mitochondria in a cell varies proportionately with energy demand. The high energy demands of neurons render them intolerant of mitochondrial dysfunction. The quantity, quality and localisation of mitochondria are all critically important for neuronal function. Mitochondrial morphology is determined by a balance between continuous fusion, which allows mitochondria within a cell to support each other, and fission, the fragmentation of mitochondria that plays an important role in apoptosis. Changes in mitochondrial dynamics are found in many neurodegenerative diseases, including ALS, and it has been postulated that the imbalance of mitochondrial fusion/fission is associated with disease-related mitochondrial dysfunction.92 ,96 Mitochondrial fragmentation has been described in the presymptomatic transgenic mouse97 and is an early feature of human ALS.93
The mtDNA molecule is small, encoding for 13 proteins, and is highly susceptible to mutations.98 Because mitochondrial renewal is a very active process, mtDNA accumulates mutations much faster than nuclear DNA, so that pathogenic mutations can affect a varying proportion of the many mtDNA molecules—from 1% to 100%.99 A mutation could be advantageous in some environments but detrimental in others and so forming part of the genetic basis underlying complex disorders such as ALS.100 Small non-coding microRNAs (miRNAs) have emerged as the key in regulating gene expression and their dysregulation in neurodegeneration. Alteration of miRNA-mediated regulatory activity potentially upsets the delicate balance required for neuronal cell development and survival, thereby contributing to disease onset and progression.101
Conclusions: implications for research and therapy
We postulate that ALS shares commonality with other neurodegenerative disorders in which there is a compelling body of evidence to indicate that the onset of clinical symptoms is preceded by a long presymptomatic period. Such a period may last for years or possibly decades, with downstream events that exceed the threshold for the emergence of clinical symptoms becoming evident only years after the pathobiological disease process commenced. As stressed by Benatar and Wuu,18 confirmation of this is likely to have profound implications for understanding disease biology, uncovering environmental risk factors, developing effective therapies and even disease prevention.
Genetic studies of late-onset neurodegenerations, including ALS, have received much attention in the last decade, but the link between manifestation of the disease phenotype and altered biochemistry and cellular biology detected in blood, cerebrospinal fluid (CSF) or through imaging, as well as miRNA and epigenetic changes, remains obscure.102 ,103 We suggest that many different biomolecular events may impact normal development in such a way that the disease only becomes clinically apparent when intrinsic compensatory mechanisms break down, perhaps decades after their onset. The processes involved are complex, interactive and progressive. The clinical syndrome of ALS becomes evident when neuronal and also possibly astroglial metabolism is overwhelmed by the accumulation of biological abnormality, especially involving energy kinetics, until a ‘tipping point’ is reached. Stress imposed by the difficulty of metabolising proteinaceous waste products, shown by TDP-43 accumulation in the proteasome and cytoplasm, is a marker of the underlying, but currently poorly understood abnormality. The disease begins clinically when the cell ‘falls over a cliff’ into an irreversible terminal cascade, leading to cell death.
It therefore follows that the current failure of therapies to effectively modify ALS may largely reflect the long time elapsed between the onset of the pathological process and the onset of overt symptomatic disease. It therefore becomes imperative to identify the primary targets of disease-causing proteins in this preclinical stage by establishing presymptomatic diagnostic tools to identify those at high risk of developing ALS.104 Furthermore, understanding the presymptomatic disease state is essential to identifying compensatory mechanisms which allow apparently normal brain functioning, despite ongoing neurodegeneration. A lengthy presymptomatic period with compromised cellular and associated neural network dysfunction, possibly arising in the perinatal period, opens a potentially important window for neuroprotective intervention that might allow rescue of dysfunctional but not yet dead neurons. It is even possible that many of the agents previously trialled, which have failed to show benefit in overt ALS, if given very early, may have neuroprotective properties. Developmental aspects in the context of the ALS clinical history and quantitating the impact of external environmental features, having proved useful in understanding autistic spectrum disorders, may in turn yield further critical insight, specifically concerning the optimal time to introduce potential neuroprotective therapy.105
References
Footnotes
-
Contributors Each author contributed equally in the concept, writing and reviewing of this manuscript.
-
Competing interests None.
-
Provenance and peer review Commissioned; externally peer reviewed.