Article Text
Abstract
Introduction The management of short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) and short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms (SUNA) remains challenging in view of the paucity of data and evidence-based treatment recommendations are missing.
Methods In this single-centre, non-randomised, prospective open-label study, we evaluated and compared the efficacy of oral and parenteral treatments for SUNCT and SUNA in a real-world setting. Additionally, single-arm meta-analyses of the available reports of SUNCT and SUNA treatments were conducted.
Results The study cohort comprised 161 patients. Most patients responded to lamotrigine (56%), followed by oxcarbazepine (46%), duloxetine (30%), carbamazepine (26%), topiramate (25%), pregabalin and gabapentin (10%). Mexiletine and lacosamide were effective in a meaningful proportion of patients but poorly tolerated. Intravenous lidocaine given for 7–10 days led to improvement in 90% of patients, whereas only 27% of patients responded to a greater occipital nerve block. No statistically significant differences in responders were observed between SUNCT and SUNA. In the meta-analysis of the pooled data, topiramate was found to be significantly more effective in SUNCT than SUNA patients. However, a higher proportion of SUNA than SUNCT was considered refractory to medications at the time of the topiramate trial, possibly explaining this isolated difference.
Conclusions We propose a treatment algorithm for SUNCT and SUNA for clinical practice. The response to sodium channel blockers indicates a therapeutic overlap with trigeminal neuralgia, suggesting that sodium channels dysfunction may be a key pathophysiological hallmark in these disorders. Furthermore, the therapeutic similarities between SUNCT and SUNA further support the hypothesis that these conditions are variants of the same disorder.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) and short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms (SUNA) are considered separate clinical entities encompassed within the trigeminal autonomic cephalalgias (TACs) group under the umbrella term ‘Short-lasting unilateral neuralgiform headache attacks’ (SUNHA).1 Given the rarity of SUNCT and SUNA, there is sparse literature on their clinical presentation, underlying pathophysiological mechanisms and response to treatments. A recent prospective comparative study refined the SUNCT and SUNA clinical phenotype in a large series of patients, confirming key overlapping characteristics with other TACs but also highlighting clinical similarities with trigeminal neuralgia (TN).2 It has been postulated that these shared clinical similarities may be driven by cross-talk between impaired functioning regions considered pivotal in the pathophysiology of TACs, such as the posterior hypothalamic area and structurally abnormal preganglionic trigeminal sensory root due to a vascular contact.3–5 In view of the clinical and pathophysiological similarities, several treatments known to be effective in other TACs and TN have been tried in SUNCT and SUNA.6 However, the current evidence is limited to small case series and one small randomised placebo-controlled trial, preventing robust treatment recommendations in clinical practice.7–11
The aim of this study was to describe the efficacy outcomes of oral and parenteral treatments used in our practice to treat a large series of SUNCT and SUNA patients. In addition, we pooled our results together with the available published data in single-arm meta-analyses to synthesise the available published data and derive a treatment algorithm.
Methods
Study design and study population
This was a single-centre, non-randomised, prospective open-label study conducted in consecutive patients diagnosed by the headache team with SUNCT and SUNA between 2007 and 2014. Diagnosis was based on the criteria of the International Classification of Headache Disorders (ICHD-2 and ICHD-3 beta).12 13 With publication of the ICHD-3 criteria, we subsequently ensured that all patients included in the study fulfilled these criteria.1 When a treatment was prescribed in clinic, clinical details were collected by two of the authors (MM and GL) directly from patients at the start of the treatment, using a semistructured standardised questionnaire. The questionnaire was designed to capture: headache characteristics including attack frequency, severity and duration at baseline and at follow-up when treatment outcome was evaluated, name of the treatment, maximum dose reached, treatment duration, treatment outcome, side effects and reasons for discontinuation (when applicable). Data were entered into a spreadsheet using Statistical Package for the Social Sciences (SPSS) V.21 (IBM SPSS Statistics). To assure quality of the data, the data collected in the questionnaires were compared against the ones of the spreadsheet by another member of the headache team. For oral treatment trials, face-to-face or telephone follow-up assessments were scheduled at 3 months to establish treatment efficacy and tolerability, and subsequently at six and twelve months, to established consistency of response. For injectable treatment trials, telephone follow-up was arranged at 6 weeks after the treatment was performed or the patient was discharged from the ward, to established efficacy and tolerability; subsequent 3-month, 6-month and 12-month follow-ups were organised to establish consistency of response.
Treatment regimens
The oral medications assessed in this study included treatments for which there were initial published evidence of efficacy, namely lamotrigine, carbamazepine, oxcarbazepine, topiramate, gabapentin and pregabalin8 9 11 14 and treatments with no reported evidence of efficacy namely duloxetine, lacosamide and mexiletine. The rationale for testing these medications in SUNCT and SUNA came from evidence of efficacy in other neuropathic and neuralgiform disorders.15–17 Standardised drug titration protocols were used for all patients to ensure homogeneity of data. The transitional preventive treatments evaluated in this study were greater occipital nerve blocks (GONB) and intravenous lidocaine. The GONB protocol used in this study consisted of an injection of a mixture of methylprednisolone 80 mg and 2 mL of 2% lidocaine in the suboccipital area.18 The intravenous lidocaine protocol used in this study is described elsewhere.19
Data collection and treatment outcomes
In the absence of standardised diaries designed to capture the multiple (sometimes hundreds) short-lasting daily SUNHA attacks, patients’ estimate of change in attacks frequency, severity and duration from baseline to after exposure to a certain treatment, was used to evaluate treatments efficacy. In patients with countable attacks, a daily attack diary, normally used to capture frequency of attacks in cluster headache (CH) was used to guarantee a certain degree of objectiveness.
Headache improvement was defined as reduction in daily attack frequency and/or intensity and/or duration. Treatment improvement was classified as: mild (<50% improvement), good (50%–90% improvement) and excellent (91%–100% improvement). Responders were defined as those patients who obtained a good or excellent improvement from a given treatment compared with baseline. Treatment failure was defined as lack of headache improvement or treatment discontinuation due to tolerability issues. Treatments outcomes were also compared between SUNCT and SUNA patients, looking for significant differences. Most treatments were tried in monotherapy, though sometimes combination of two treatments was required when the first medication had produced a partial but meaningful response. We ensured that when a treatment was added in polytherapy, the baseline drug dosage was kept steady to ensure meaningful assessment of the second added medication. Some patients had tried some of the medications we evaluated in this study before attending our clinic. Unless the details of the treatment were clearly documented, the drug/s were re-explored following our titration paradigm.
Patients provided written consent. This study is derived from Dr Lambru’s PhD thesis in the University College London repository.20
Statistical analysis
All data were analysed using SPSS V.21 (IBM SPSS Statistics). χ2 test and Fisher’s exact test were used to compare categorical variables. Paired sample t-test was used to compare numerical variables. All reported p values are two sided and a significance level less than 5% was considered significant. A Bonferroni adjustment was applied to p values to control the type I error inflation as a result of multiple testing.
Meta-analysis
Single arm meta-analysis of the medical treatment of SUNCT and SUNA was also conducted. Eligible studies were identified from a database search of Embase and Medline from their inception to January 2020 using PubMed and Ovid. The terms “SUNCT”, “SUNA”, “Short-lasting neuralgiform headache attacks” were used as search keywords. Additionally, we hand-searched the reference lists of retrieved articles and reviews on the subject. Titles and abstracts of reports identified in the search were screened, and full text was reviewed when there was insufficient information in the abstract to determine eligibility. Eligible studies were required to have: a case series, audit, cohort, case–control, or randomised controlled trial design; include at least five participants diagnosed with SUNCT and/or SUNA; investigate medical preventive or transitional treatments in SUNCT/SUNA; and be published in English. The following data were extracted from each included study: first author, year of publication, country of origin, number of participants number of SUNCT and SUNA, method of data collection, treatments efficacy outcomes, medication trial duration, different dose protocols and presence of missing data. We attempted to use 50% improvement in headache frequency and/or intensity and/or duration as definition of responders. In cases where this was not available, the respective reports’ definition of clinically meaningful response was used to define responders. The final reported timepoint for the efficacy outcome was used. In cases where there were duplicate publications of the same study population, we included only the most recent publication.
Data analyses were based on aggregated data. We extracted data from figures and tables and converted data when necessary. We calculated responder proportions with 95% CI using a fixed-effects inverse variance model with a double arcsine transformation.21 Heterogeneity was quantified by use of the I2 statistic.22 We conducted separate analyses for SUNCT, SUNA and the two entities lumped together. All analyses were made using R V.3.6.0 (The R Foundation for Statistical Computing) with the open-source package meta V.4.11-0. We summarised the characteristics of all included studies and carried out a risk of bias assessment according to the Risk Of Bias in Non-randomized Studies-of Intervention (ROBINS-I) tool.23
Results
The study cohort comprised 161 SUNCT and SUNA patients. The baseline demographic and clinical characteristics of the cohort are summarised in table 1.
Demographic and clinical features of the short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) and short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms (SUNA) cohorts
Oral preventive treatments with previous evidence in SUNCT and SUNA
Table 2 outlines the number of patients, the mean doses, therapeutic outcomes for each medication tried, as well as the proportion of patients who discontinued the medications because of side effects.
Doses, duration of trials, therapeutic outcome and discontinuation of oral preventive treatments in short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) and short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms (SUNA)
Lamotrigine
Seventy-six per cent of patients (n=97/128) (SUNCT: 73%; SUNA 72%, p=0.935) reported some degree of headache improvement with lamotrigine. Of these, 56% (n=71/128) were considered responders (SUNCT: 54%; SUNA: 58%, p=0.620). Lamotrigine was tolerated by 82% of patients. Six SUNCT and three SUNA patients needed to exceed the British National Formulary (BNF) recommended doses of 400 mg daily to obtain a beneficial effect.
Oxcarbazepine and carbamazepine
Oxcarbazepine led to an improvement in 71% of patients (n=45/63) (SUNCT: 69%; SUNA: 74%, p=0.689), of whom 46% were considered responders (n=29/63) (SUNCT: 45%; SUNA=47%, p=0.859). One SUNCT one SUNA patient needed to exceed the BNF recommended dose of 2400 mg daily to obtain a beneficial effect.
Fifty per cent of patients (n=42/84) reported improvement with carbamazepine (SUNCT: 37%; SUNA: 63%, p=0.024, threshold for significance after Bonferroni correction is 0.006). Of these, 26% were considered responders (SUNCT: 16%; SUNA: 37%, p=0.042, threshold for significance after Bonferroni correction is 0.006). One SUNA and no SUNCT patient needed to exceed the BNF recommended dose of 1600 mg daily to obtain meaningful improvement. A similar proportion of patients discontinue oxcarbazepine (17%) and carbamazepine (20%) because of side effects.
Topiramate
Topiramate led to improvement in 47% of patients (n=36/76) (SUNCT: 54%; SUNA: 36%, p=0.120), of whom 25% (n=19/76) were considered responders (SUNCT: 33%; SUNA: 11%, p=0.028, threshold for significance after Bonferroni correction is 0.006). Three SUNCT and no SUNA patients needed to exceed the BNF recommended dose of 400 mg daily to obtain improvement. Twenty-four patients (32%) discontinued the treatment because of side effects.
Gabapentin and pregabalin
Gabapentin led to improvement in 33% of patients (n=26/80) (SUNCT: 32%; SUNA: 33%, p=0.902), of whom 10% (n=8/80) were responders (SUNCT: 10%; SUNA: 10%, p=1.00). Similarly, pregabalin led to headache improvement in 30% of patients (n=19/64) (SUNCT: 31%; SUNA: 29%, p=0.863), of whom 11% of patients (n=7/64) were considered responders (SUNCT: 11%; SUNA: 11%, p=0.960). One SUNCT one SUNA patient needed to exceed the BNF recommended doses for gabapentin of 3600 mg daily to obtain a beneficial effect. Side effects led to treatment discontinuation of gabapentin and pregabalin in 14% and 21% of patients, respectively.
Oral preventive treatments with no previous evidence in SUNCT and SUNA
Duloxetine, mexiletine and lacosamide were tried in difficult-to-treat patients refractory to the drugs described above.
Duloxetine
Duloxetine led to improvement in 49% of patients (n=18/37) (SUNCT: 60%; SUNA: 35%, p=0.134), of whom 30% (n=11/37) were considered responders (SUNCT: 45%, SUNA: 12%, p=0.027, threshold for significance after Bonferroni correction is 0.006). One SUNCT patient reported a worsening of the headache while on duloxetine. Tolerability issues led to drug discontinuation in 29% (n=8/28) of patients.
Mexiletine
Mexiletine led to a headache improvement in 60% (n=9/15) of patients. Seventy-five per cent of SUNCT and 43% of SUNA patients reported a headache improvement (p=0.205), of whom 33% were considered responders with no significant differences between SUNCT and SUNA (p=0.714). Seven SUNCT and five SUNA patients needed to exceed the BNF recommended dose of 500 mg daily, though only six out of seven SUNCT and three out of five SUNA benefited from the increased doses. Side effects led to drug discontinuation in six patients (40%).
Lacosamide
Two out of six patients who tried lacosamide and for whom we had reliable outcome data reported a headache improvement (33%). Both patients were considered responders. One SUNCT and one SUNA patient needed to exceed the BNF recommended dose of 300 mg daily to obtain a beneficial effect. Two patients could not tolerate the medication and two patients did not respond.
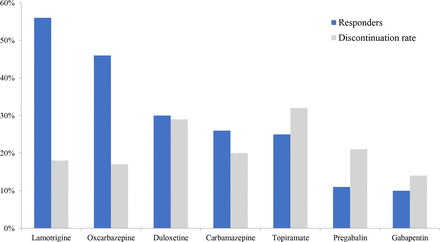
Figure 1 shows a comparison of the response rates and discontinuation rates due to adverse effects between the various oral medications tried in our cohort.
Responder and discontinuation rates secondary to adverse effects for oral medical treatments in SUNCT and SUNA. SUNCT: Short-lasting Unilateral Neuralgiform headache attacks with Conjunctival injections and Tearing; SUNA: Short-lasting Unilateral Neuralgiform headache attacks with autonomic symptoms. * Mexiletine and lacosamide data were not included due to small sample size.
Parenteral treatments used as transitional therapies
Intravenous lidocaine
Table 3 summarises the outcome and duration of improvement of intravenous lidocaine. Ninety per cent of patients were considered responders to intravenous lidocaine (n=52/58) (SUNCT: 91%, n=32; SUNA: 87%, n=20, p=0.898). Most responders (29/52) experienced the maximum benefit with an average infusion speed of 120 mg/hour. In about half of responders, the attacks recurred immediately after the end of the infusion period, whereas in the remaining responders, the mean duration of the improvement was 22.1 days (range: 3–90 days). Three patients discontinued the infusion because of side effects, such as cognitive slowing and paranoid thoughts.
Summary of treatment responses using intravenous lidocaine and greater occipital nerve blocks (GONB) as transitional treatment in short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) and short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms (SUNA)
Greater occipital nerve block
Table 3 summarises the outcome and duration of response of a single GONB tested in our cohort. Of 78 patients, 66 received a unilateral GONB, whereas 12 patients received bilateral injections in view of their unilateral side-alternating attacks. Headache improvement after the injection was reported by 37% (n=29/78) of patients lasting for a mean of 38.4±34.7 days (range 1–150 days). Four patients reported a clinical improvement lasting between 1 and 7 days (14%), 15 patients between 10 and 30 days (52%), 9 patients between 37 and 90 days (31%) and 1 patient (4%) for 5 months. Twenty-seven per cent of patients were considered responders, reporting a mean duration of response of 41.3±36.3 days with no significant differences between SUNCT and SUNA (p=0.390). Of the non-responders, 18% (n=9/49) reported a worsening of headache after the GONB for a mean of 12.7±8.1 days. The procedure was well tolerated by all other patients and no other adverse events were reported.
Single arm meta-analysis: pooled results
Out of 96 articles, 5 studies with a total of 154 patients, in addition to the present study, met eligibility criteria and were included in the meta-analysis.4 11 14 24 25 All pooled weighted proportions of responders are provided in table 4. Lamotrigine showed the highest percentage of responders among all the treatments for both SUNCT (0.64; 95% CI 0.50 to 0.77) and SUNA (0.46; 95% CI 0.24 to 0.68) (figure 2). No statistically significant differences in responder proportions between SUNCT and SUNA were observed, apart from topiramate, which was more effective in SUNCT than SUNA patients (figure 3). The I2-statistics suggests moderately large heterogeneity, which is likely explained by differences in study designs. Characteristics of the included studies and risk-of-bias assessments are provided in online supplemental table 1 (online supplemental material).
Supplemental material
Pooled weighted responder proportion for all treatments reported in at least one other study with five or more participants
Forest plot of percentage of responders to lamotrigine in SUNCT and SUNA. SUNCT short-lasting unilateral neuralgiform attacks with conjunctival injection and tearing (SUNCT) and SUNA short-lasting unilateral neuralgiform headache attacks with autonomic symptoms (SUNA); CI = confidence interval.
Pooled weighted responder proportions with 95% CIs for all drugs analysed. SUNA, short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms; GONB: greater occipital nerve block; SUNCT, short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing.
Discussion
Our data coming from the largest series of patients so far analysed confirmed that among oral preventive medicines lamotrigine is the treatment of choice for SUNCT and SUNA.14 Unlike the findings of a small study where lamotrigine was reported to be effective in episodic but not in chronic SUNCT/SUNA patients, our data confirm the clinical usefulness of this medication also in the chronic subtype.4 The proportion of responders in our study was similar to the one of an audit of 29 SUNCT patients where lamotrigine was found to be efficacious in 62% of them.11 However, unlike the poor outcome observed in their SUNA patients, our data confirmed that lamotrigine is very effective also in SUNA with similar proportion of responders to SUNCT.
Oxcarbazepine emerged as a more effective drug compared with carbamazepine. Indeed, our evidence supports the view that oxcarbazepine should be considered as the second option in patients not responding to, or tolerating lamotrigine, or as an add-on treatment to lamotrigine, when lamotrigine alone does not provide sufficient control of symptoms. Our outcomes are in contrast to a previous audit where carbamazepine was more effective than oxcarbazepine, the latter showing a very low percentage of responders, possibly due to the small sample size.11 Though oxcarbazepine is a structural derivative of carbamazepine, its mechanisms of action have been found to be partly different from carbamazepine mainly by blocking sodium channels at a lower dose compared with carbamazepine and by modulating different types of calcium channels, possibly explaining their different efficacies.26
Topiramate was shown to be more effective in SUNCT compared with SUNA patients in a recent audit. The percentage of responders in a small group of SUNCT was 40% when the outcomes were controlled with placebo.11 Our outcomes showed a lower percentage of responders, with only one fourth of SUNCT and even a lower percentage of SUNA patients who benefited greatly from topiramate. It is possible that its poor tolerability profile has contributed to this outcome.
Gabapentin was found to be effective in a 38% of SUNCT and 39% of SUNA patients, without any significant differences between them.11 These results were not confirmed in our study, where only approximately 10% of patients benefited significantly from this drug. Similar outcomes were observed in patients treated with pregabalin, suggesting that perhaps both drugs might be helpful mainly in combination with another drug, rather than in monotherapy.9
The proportion of responders to duloxetine was higher than the proportion of responders to topiramate and carbamazepine, supporting the view that this medication may play an important role in management of SUNHA in patients who fail lamotrigine or oxcarbazepine. Mexiletine and lacosamide displayed a poor tolerability profile, with high discontinuation rates. Nonetheless, given that both medications were tried in a refractory group of patients, these drugs might still be considered as an option before more invasive approaches are offered.27–29
High doses of medications were often required to obtain a meaningful treatment outcome in our patients. This may reflect a referral bias, given that our centre receives among the most complex and difficult-to-treat patients in the UK. It has also been shown that chronic SUNCT/SUNA may respond less well to medications compared with the episodic forms.4 Furthermore, the chronic subtypes of these conditions often do not respond sufficiently well to medical treatments, requiring surgical approaches.27–29 These and our findings suggest that the chronic subtypes of SUNCT and SUNA maybe a particularly difficult-to-treat groups of headache disorders; hence, high doses may be required before considering a medication ineffective. The side effect profile of these drugs was often dose related. Some patients only had side effects at the higher doses, while others developed side effects at lower doses and they worsened gradually with dose titration. Some patients had significant side effects at low doses while others tolerated high doses without any side effects at all.
Considering both the efficacy and side effect profile of the treatments tested in this study, we propose a three-tier algorithm of oral preventive treatments for SUNCT and SUNA for clinical practice purposes (table 5).
Three-tier algorithm of oral preventive treatments for short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing and short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms
Our data on intravenous lidocaine are in line with the results of previous series, showing excellent, although short-lived, response in the vast majority of SUNCT and SUNA patients.19 Other authors administered lidocaine either as an intravenous infusion, or as a subcutaneous injection for 6–8 days. Thirteen of their 14 patients had excellent results, with most patients becoming headache free, suggesting that either route of administration could be successfully employed.4 These data suggest that intravenous lidocaine should be the treatment of choice in SUNCT and SUNA status.30
GONB holds robust evidence of efficacy in CH and some preliminary evidence of efficacy in the other TACs.31–33 Preliminary audit data of a GONB in SUNCT and SUNA showed a meaningful response in about half of the patients.11 Our findings were less impressive, showing a significant response in less than one-third of patients. A plausible explanation for the outcome discrepancies between CH and SUNHA may reside in the different response that these conditions have to corticosteroids, very effective in the former and generally ineffective in the latter.11 34 Our and previous studies highlight the substantial difference in treatment outcomes between SUNHA and the other TACs.11 35 The findings on GONB seem to further support the notion that SUNHA differs considerably in the treatment response from the other TACs.
No significant differences emerged in our study from the comparison of oral and parenteral treatments between SUNCT and SUNA. The similar treatment outcomes between the two conditions was also observed in the pooled analysis, where lamotrigine and oxcarbazepine were confirmed to be the most effective oral treatments and intravenous lidocaine the most effective transitional treatment. The meta-analysis of the pooled data demonstrated superiority of topiramate in SUNCT compared with SUNA. This may be explained by the different level of refractoriness of SUNCT and SUNA patients at the time of the topiramate trial. Seventy-four per cent (n=23/31) of SUNA patients compared with 48% (n=23/48) of SUNCT patients tried topiramate after having failed at least four treatments among lamotrigine, oxcarbazepine, carbamazepine, gabapentin and pregabalin. This indicates that a greater number of SUNA patients were more difficult-to-treat compared with SUNCT by the time they were exposed to topiramate, probably explaining this isolated difference.
Mechanisms of action of drugs effective in SUNCT and SUNA and pathophysiological implications
Among the multiple mechanisms of action, all the effective drugs in our analysis have in common the inhibition of voltage-gated sodium channels. The efficacy of sodium channels blockers in SUNHA indicates that at least in some patients, a sodium channel dysregulation could constitute a relevant pathophysiological mechanism worth exploring further. Initial evidence in TN has already shown the relevance of imbalanced sodium channels activity with downregulation of Nav1.7 and upregulation of Nav1.3.36 In view of the striking similarities of drug response between SUNHA and TN, studies exploring the role of impaired ion channels may reveal a further common denominator in the pathogenesis of these conditions.
This study has some limitations, including the lack of a placebo arm. It is possible that the outcome of certain drugs has been exaggerated, partially reflecting a placebo effect. To try and minimise this, we used 50% cut-off improvement as a clinically robust outcome measure, to define responders. This, along with the persistent improvement in patients with a protracted preceding chronic phase, may have reduced the likelihood that reported responses are due to placebo. Caveats in setting up placebo-controlled trials in these conditions include difficulty in enrolling patients due to the rarity and severity of SUHNA, which will lead to underpowered studies. Moreover, conducting placebo-controlled trials in such excruciating conditions poses ethical issues related to the use of a placebo compound and the long duration of the placebo phase due to gradual drug titration.
Conclusion
SUNCT and SUNA seem to display similar responses to pharmacological treatments. The higher proportion of SUNCT responders to topiramate is likely to have resulted from the different level of refractoriness between SUNCT and SUNA patients at the time of trial. Lamotrigine should be considered the drug of choice for the management of SUNCT and SUNA. Oxcarbazepine, duloxetine and topiramate can be useful options for patients who fail to respond to lamotrigine or as add-on options. Intravenous lidocaine is an extremely effective treatment for patients with frequent, severe attacks, but it may not be available in every hospital. Conversely, GONB may only be effective in a small proportion of patients. The efficacy of sodium channels blockers raises the possibility that one of the biological hallmarks of SUNHA may be sodium channel dysfunction.
Acknowledgments
The authors would like to thank their headache specialist nurses for their help with completion of the clinical database and management of patients. They also thank the patients and their families for their help with this project.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @manjit_matharu
Contributors GL: study concept, collection, analysis, and interpretation of data, drafting and revision of the manuscript. AS: analysis and interpretation of data, drafting and revision of the manuscript. KR: statistical analysis and interpretation of data, revision of the manuscript. SL: collection and quality assurance of data, revision of the manuscript. ET: study concept, interpretation of data and manuscript revision. MSM: study concept, recruitment of subjects, interpretation of data and manuscript revision.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests GL has received speaker honoraria, funding for travel and has received honoraria for participation in advisory boards sponsored by Allergan, Novartis, Eli Lilly and TEVA. He has received speaker honoraria, funding for travel from electroCore, Nevro Corp. and Autonomic Technologies. AS is cofounder of Nordic Brain Tech, a company developing a non-pharmacological biofeedback treatment for migraine and holds a pending patent application relating to the company’s product. KR has nothing to declare. SL has received payment for attending advisory meetings and development of presentation from Allergan Novartis, Eli Lilly and TEVA. ET: is a cofounder and shareholder of Nordic Brain Tech AS and Palion Medical AS. He serves on advisory board for Eli Lilly and Novartis and TEVA. He has received speaker honoraria from Eli Lilly, Novartis, Allergan and TEVA. MSM serves on the advisory board for Abbott, Allergan, Eli Lilly, Medtronic, Novartis, TEVA; has received payment for the development of educational presentations from Allergan, electroCore, Eli Lilly, Medtronic, Novartis, and TEVA; and, has received research grants from Abbott, electroCore and Medtronic.
Patient consent for publication Not required.
Ethics approval The study was approved by Northwick Park Hospital Research Ethics Committee, London, UK (REC no:11/LO/1709).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request. The data that support the findings of this study are available upon reasonable request to the corresponding author.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.